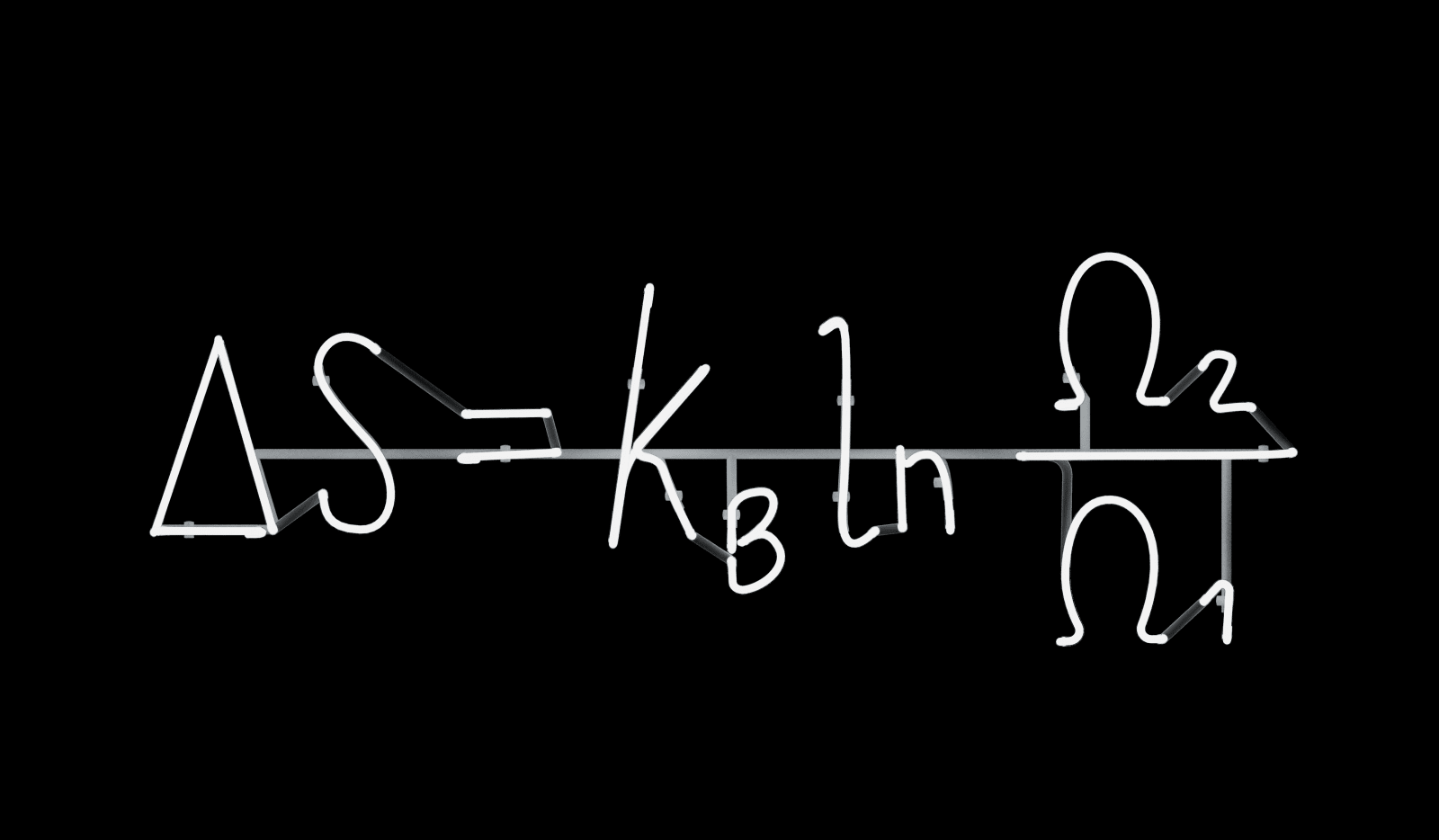Andrea Galvani Italy, b. 1973
Boltzmann Change in Entropy, 2019
6500K neon, white blown glass, metal structure
32 x 94 x 7 cm
Edition of 3
Entropy is known as the arrow of time. It predicts the ultimate heat-death of the Universe, and provides the driving force for the development of structure as well as decay....
Entropy is known as the arrow of time. It predicts the ultimate heat-death of the Universe, and provides
the driving force for the development of structure as well as decay. Entropy is surely one of the most intruguing and misunderstood concepts in physics. Both the quantity and the word were introduced by Rudolph Clausius (1822-1888), who presented a measurement for the irreversible process of heat in only one direction. He named this process entropy, from the ancient Greek word for transformation: ἠ τροπὴ. One problem was that Clausius conceptualized heat as a physical fluid called caloric. It took a revolution to understand the reality of entropy and heat, and that revolution started with Ludwig Boltzmann (1844-1906). Boltzmann began to see what was behind Clausius’ equations, throwing us into one of our most dizzying dives towards understanding the intimate grammar of the Universe. Andrea Galvani’s Boltzmann Change in Entropy reveals that the difference between the past and the future is simply entropy.
Heat is a concept that seems simple and familiar to us. It’s a sensation we know intimately: the feeling
of the Sun when it kisses our skin; the breath of a flame that both beckons and warns; a cup of tea in the afternoon; a fever that makes us sweat. Boltzmann, one of the few at the end of the nineteenth century who believed in the existence of atoms, understood that heat is a state in which atoms move quickly. A substance is cold when atoms and molecules move more slowly. A substance is hot when they more more rapidly. Why does heat seem to flow in one direction only, from hot to cold? As strange as it may seem,
the answer is probability. It is simply more likely that when faster-moving atoms of a hot substance collide with slower-moving atoms of a cold substance, the hotter atoms leaves a little bit of their energy, rather than vice versa. Energy is conserved, but tends to get distributed in a way that equalizes the temperature of objects in contact. It’s not impossible for a hot body to get hotter through contact with a cold body,
it’s just improbable. Introducing probability to the core of physics and using it to explain the basis of thermodynamic interactions was initially considered absurd. No one took Boltzmann seriously. In a state of deep depression, he committed suicide. He never witnessed the subsequent universal recognition of the validity of his ideas.
the driving force for the development of structure as well as decay. Entropy is surely one of the most intruguing and misunderstood concepts in physics. Both the quantity and the word were introduced by Rudolph Clausius (1822-1888), who presented a measurement for the irreversible process of heat in only one direction. He named this process entropy, from the ancient Greek word for transformation: ἠ τροπὴ. One problem was that Clausius conceptualized heat as a physical fluid called caloric. It took a revolution to understand the reality of entropy and heat, and that revolution started with Ludwig Boltzmann (1844-1906). Boltzmann began to see what was behind Clausius’ equations, throwing us into one of our most dizzying dives towards understanding the intimate grammar of the Universe. Andrea Galvani’s Boltzmann Change in Entropy reveals that the difference between the past and the future is simply entropy.
Heat is a concept that seems simple and familiar to us. It’s a sensation we know intimately: the feeling
of the Sun when it kisses our skin; the breath of a flame that both beckons and warns; a cup of tea in the afternoon; a fever that makes us sweat. Boltzmann, one of the few at the end of the nineteenth century who believed in the existence of atoms, understood that heat is a state in which atoms move quickly. A substance is cold when atoms and molecules move more slowly. A substance is hot when they more more rapidly. Why does heat seem to flow in one direction only, from hot to cold? As strange as it may seem,
the answer is probability. It is simply more likely that when faster-moving atoms of a hot substance collide with slower-moving atoms of a cold substance, the hotter atoms leaves a little bit of their energy, rather than vice versa. Energy is conserved, but tends to get distributed in a way that equalizes the temperature of objects in contact. It’s not impossible for a hot body to get hotter through contact with a cold body,
it’s just improbable. Introducing probability to the core of physics and using it to explain the basis of thermodynamic interactions was initially considered absurd. No one took Boltzmann seriously. In a state of deep depression, he committed suicide. He never witnessed the subsequent universal recognition of the validity of his ideas.